Behind the Bubbles:
The Science of Sparkling Soda

Some natural springs produce naturally carbonated water. One famous example is the Selters spring in Germany, from which the term “seltzer” is derived.
Sparkling sodas have long been a beloved beverage choice, captivating our taste buds with their fizzy, effervescent quality. But have you ever wondered about the science behind those delightful bubbles? Let’s dive into the fascinating process of carbonation and explore what makes sparkling sodas so fizzy and fun.

The Basics of Carbonation
At its core, carbonation is the process of dissolving carbon dioxide (CO2) gas into a liquid under high pressure. This is what creates the signature bubbles in sparkling sodas. When the pressure is released, such as when you open a bottle or can, the CO2 escapes from the liquid, forming bubbles that rise to the surface.

How Is Carbonation Achieved?
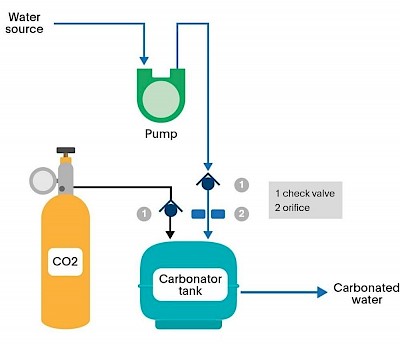
There are several methods to carbonate beverages, but the most common technique involves injecting CO2 into the liquid under high pressure. Here’s a step-by-step look at how it’s typically done in the beverage industry:
- Mixing: The base liquid, which could be water or a flavored mix, is prepared.
- Chilling: The liquid is chilled, as cold liquids can hold more CO2 than warm ones.
- Pressurizing: CO2 is injected into the liquid at high pressure in a sealed environment.
- Agitation: The liquid is agitated to ensure the CO2 is evenly distributed.
- Bottling: The carbonated liquid is then bottled or canned under pressure to maintain the carbonation until the container is opened.
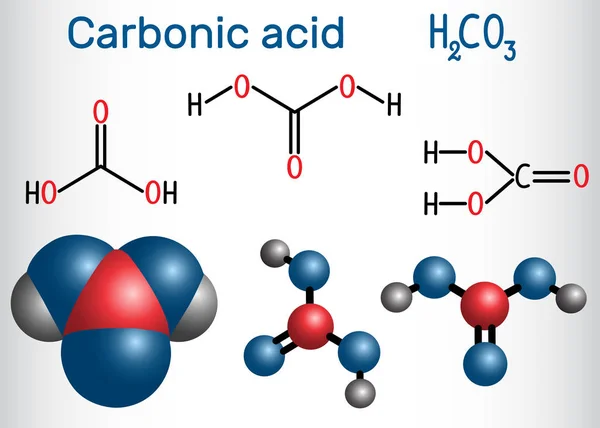
The Role of Carbonic Acid
When CO2 dissolves in water, it forms carbonic acid (H2CO3). This weak acid is what gives sparkling sodas their slight tanginess. The carbonic acid quickly breaks down back into CO2 and water, but the transient presence of carbonic acid plays a crucial role in the sensory experience of drinking a sparkling soda.
The Fun of Fizz
The bubbles in sparkling sodas not only provide a delightful texture but also enhance the flavor experience. The CO2 gas is released as the bubbles burst, carrying aromatic compounds to the nose and enhancing the perception of flavor. This multisensory experience is part of what makes sparkling sodas so enjoyable.

Experimenting with Carbonation
For those who love a bit of science at home, experimenting with carbonation can be a fun and educational activity. Home carbonation kits are widely available and allow you to carbonate your own beverages. By adjusting the CO2 levels, you can see firsthand how different levels of carbonation affect the taste and texture of your drink.

Conclusion
Understanding the science behind sparkling sodas deepens our appreciation for these fizzy delights. From the careful process of carbonation to the sensory joy of popping bubbles, each sip of a sparkling soda is a testament to both science and enjoyment. So next time you crack open a cold, fizzy soda, take a moment to savor the bubbles and the fascinating process that brought them to your glass.
By exploring the science behind the bubbles, we not only enjoy our drinks more but also gain a greater appreciation for the craftsmanship that goes into every bottle. Cheers to the fizzy fun of sparkling sodas!
